Key Takeaways
- Non-invasive PGT-A analyzes embryo DNA without biopsy
- Uses spent culture media collected during IVF
- Eliminates physical manipulation of the embryo
- May support safer embryo selection in surrogacy
- Still considered an emerging technology in fertility care
Preimplantation Genetic Testing for Aneuploidy (PGT-A) has become a valuable tool in IVF, helping identify embryos with the correct number of chromosomes. Traditionally, PGT-A requires an embryo biopsy—a procedure that, while generally safe, involves removing cells from the embryo.
Non-invasive PGT-A (niPGT-A) represents a promising evolution. By analyzing spent culture media—the fluid in which embryos grow—genetic information can be gathered without touching the embryo itself. For intended parents and gestational surrogates, this innovation offers a potentially gentler approach to embryo selection.
What Is Non-Invasive PGT-A?
Non-invasive PGT-A is a genetic testing method that examines cell-free DNA released by the embryo into the culture medium during development.
What Is Spent Culture Media?
Spent culture media is the nutrient-rich fluid that surrounds the embryo in the lab. As embryos grow, they naturally release small amounts of DNA into this medium, which can then be analyzed.
How Non-Invasive PGT-A Works
Step-by-Step Process
- Embryos are cultured in individual droplets
- Spent culture media is collected at the blastocyst stage
- Cell-free DNA is extracted from the fluid
- Chromosomal analysis is performed
- Embryos are ranked based on genetic findings
This approach avoids embryo biopsy entirely.
Non-Invasive PGT-A vs Traditional PGT-A
Traditional PGT-A
- Requires embryo biopsy
- Involves removal of trophectoderm cells
- Highly validated and widely used
Non-Invasive PGT-A
- No embryo manipulation
- Lower procedural risk
- Still under clinical evaluation
- Results may be influenced by DNA contamination
Why This Matters in Gestational Surrogacy
In surrogacy, embryo quality plays a critical role in:
- Implantation success
- Pregnancy stability
- Reducing miscarriage risk
Non-invasive PGT-A offers an added layer of reassurance by supporting embryo selection without increasing physical intervention, aligning well with surrogate safety priorities.
Who May Benefit Most from Non-Invasive PGT-A?
Non-invasive PGT-A may be considered when:
- Intended parents prefer minimal embryo manipulation
- There are ethical or medical concerns about biopsy
- Multiple embryos require ranking
- It is used alongside traditional testing methods
It may not yet replace standard PGT-A in all cases.
Case Study
Background:
An intended parent couple pursuing surrogacy wanted genetic screening but were concerned about embryo biopsy.
Approach:
Their clinic offered non-invasive PGT-A alongside standard embryo grading.
Outcome:
- Selection of a high-quality embryo
- Successful transfer into a gestational surrogate
- Ongoing healthy pregnancy
Testimonials
Intended Parent – USA
“Non-invasive PGT-A gave us peace of mind without adding stress to the embryos.”
Gestational Surrogate – UK
“I appreciated that the embryos weren’t physically altered before transfer.”
IVF Lab Scientist
“This technology reflects where IVF is heading—toward safer, less invasive methods.”
Expert Quote
“Non-invasive PGT-A shows promise, but careful interpretation and patient selection are essential.”
— Dr. Ritu Malhotra, Reproductive Geneticist
Related Links
- Genetic Testing in IVF and Surrogacy
- PGT-A Explained
- Advanced IVF Laboratory Technologies
- Embryo Development and Grading
Glossary
- PGT-A: Preimplantation Genetic Testing for Aneuploidy
- Non-Invasive PGT-A: Genetic testing without embryo biopsy
- Spent Culture Media: Fluid surrounding embryos during culture
- Cell-Free DNA: DNA fragments released into the culture medium
- Blastocyst: An embryo 5–6 days after fertilization
Frequently Asked Questions (FAQ)
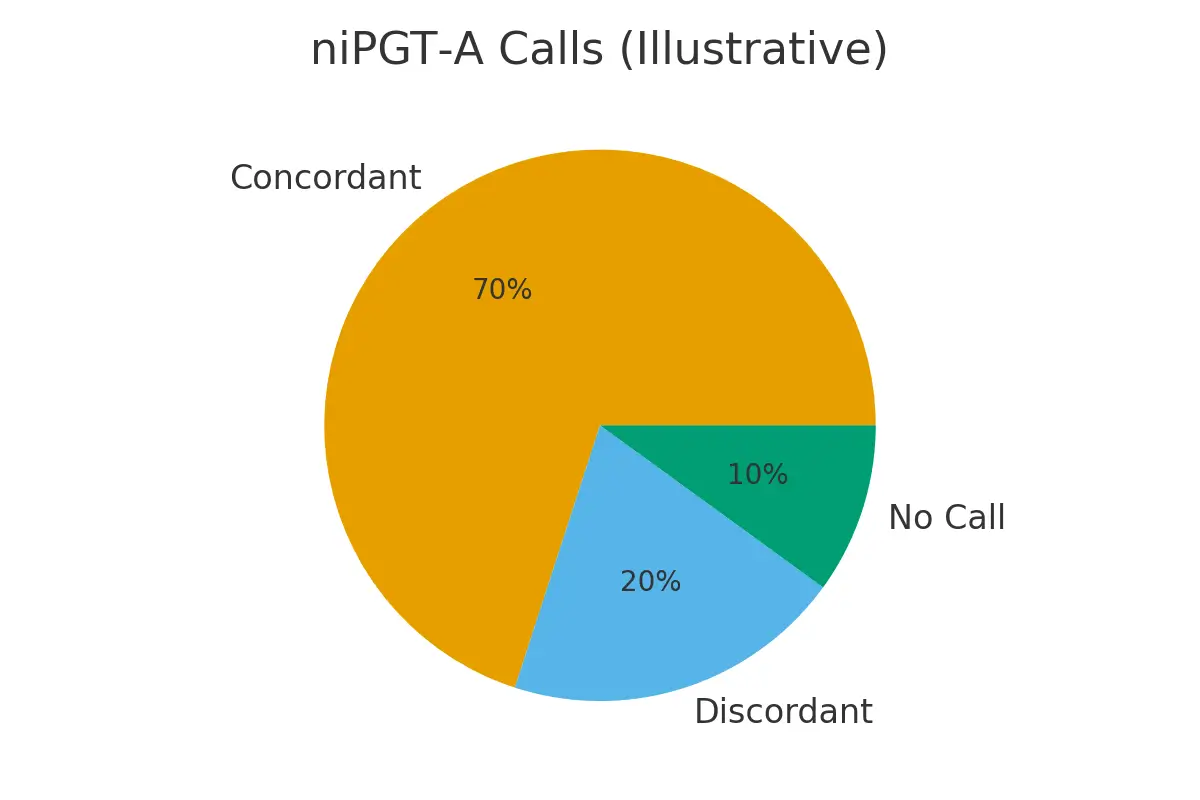
Q. Is non-invasive PGT-A as accurate as traditional PGT-A?
Ans : It shows promising accuracy but is still considered emerging compared to biopsy-based testing.
Q. Does non-invasive PGT-A harm the embryo?
Ans : No. It does not involve any embryo manipulation.
Q. Can non-invasive PGT-A replace traditional PGT-A?
Ans : Not yet. Many clinics use it as a complementary tool.
Q. Is this technology widely available?
Ans : Availability varies and is typically limited to advanced IVF labs.
Q. Does it reduce miscarriage risk?
Ans : It may help by identifying chromosomally normal embryos.
Q. Is non-invasive PGT-A suitable for surrogacy?
Ans : Yes, especially when minimizing embryo handling is a priority.
Q. What are the limitations of spent culture media testing?
Ans : Potential DNA contamination and lower DNA yield.
Q. Does it affect embryo freezing?
Ans : No. Embryos can still be frozen normally.
Q. Is donor sperm or egg DNA detected?
Ans : Only embryo-derived DNA is targeted, though contamination is monitored.
Q. Is non-invasive PGT-A expensive?
Ans : Costs vary and may be similar to or slightly lower than traditional PGT-A.
Q. Who decides whether this test is appropriate?
Ans : A fertility specialist and genetic counselor guide the decision.
Q. Is non-invasive PGT-A ethically preferable?
Ans : Some patients prefer it due to the absence of biopsy.

Dr. Kulsoom Baloch
Dr. Kulsoom Baloch is a dedicated donor coordinator at Egg Donors, leveraging her extensive background in medicine and public health. She holds an MBBS from Ziauddin University, Pakistan, and an MPH from Hofstra University, New York. With three years of clinical experience at prominent hospitals in Karachi, Pakistan, Dr. Baloch has honed her skills in patient care and medical research.